Risk of Anaphylaxis Among New Users of GLP-1 Receptor Agonists: A Cohort Study
Published in Diabetes Care, April 1, 2024 | Online publication on February 16, 2024
Authors:
Mary S Anthony![]() , Vanita R Aroda
, Vanita R Aroda![]() , Lauren E Parlett
, Lauren E Parlett![]() , Leila Djebarri, Sofia Berreghis, Brian Calingaert
, Leila Djebarri, Sofia Berreghis, Brian Calingaert![]() , Daniel C Beachler
, Daniel C Beachler![]() , Christopher L Crowe
, Christopher L Crowe![]() , Catherine B Johannes
, Catherine B Johannes![]() , Juhaeri Juhaeri
, Juhaeri Juhaeri![]() , Stephen Lanes, Chunshen Pan, Kenneth J Rothman, Catherine W Saltus, Kathleen E Walsh
, Stephen Lanes, Chunshen Pan, Kenneth J Rothman, Catherine W Saltus, Kathleen E Walsh
DOI: 10.2337/dc23-1911 | Pubmed ID: 38363873
Abstract
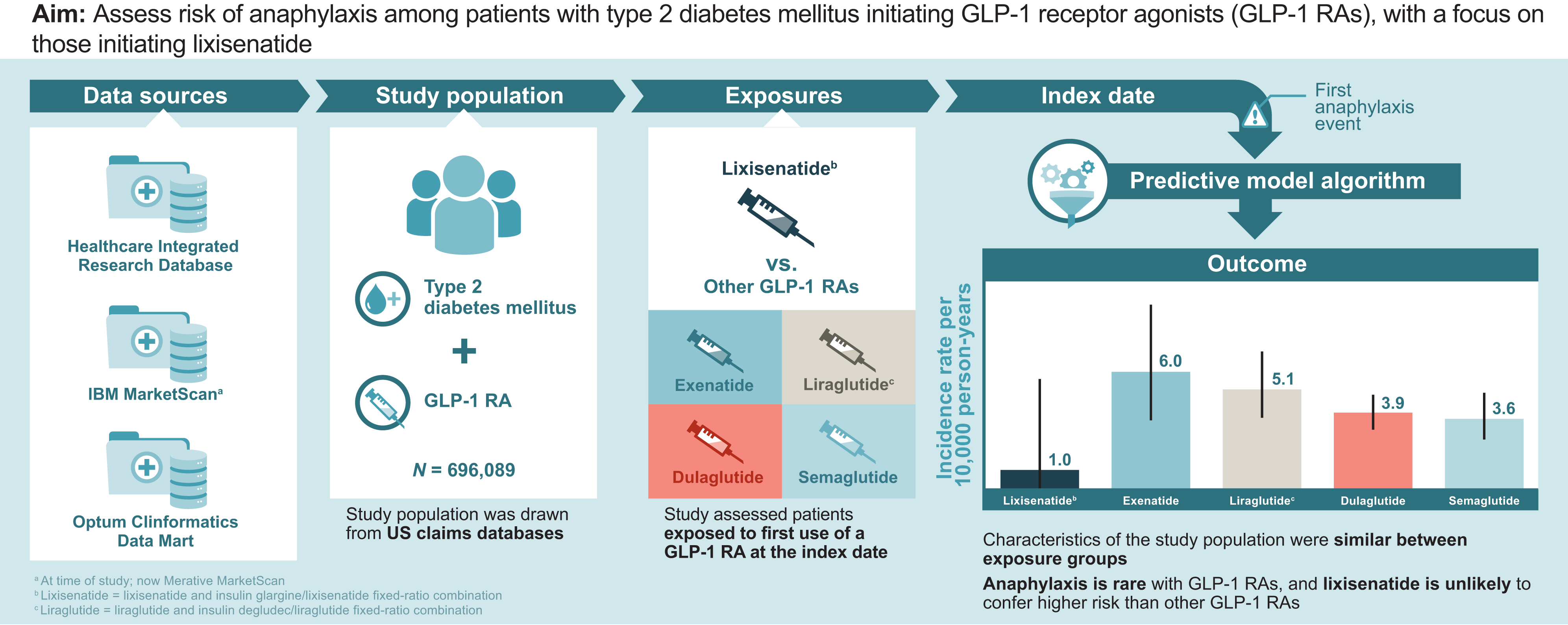
Objective: To assess risk of anaphylaxis among patients with type 2 diabetes mellitus who are initiating therapy with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), with a focus on those starting lixisenatide therapy.
Research design and methods: A cohort study was conducted in three large, U.S. claims databases (2017-2021). Adult (aged ≥18 years) new users of a GLP-1 RA who had type 2 diabetes mellitus and ≥6 months enrollment in the database before GLP-1 RA initiation (start of follow-up) were included. GLP-1 RAs evaluated were lixisenatide, an insulin glargine/lixisenatide fixed-ratio combination (FRC), exenatide, liraglutide or insulin degludec/liraglutide FRC, dulaglutide, and semaglutide (injectable and oral). The first anaphylaxis event during follow-up was identified using a validated algorithm. Incidence rates (IRs) and 95% CIs were calculated within each medication cohort. The unadjusted IR ratio (IRR) comparing anaphylaxis rates in the lixisenatide cohort with all other GLP-1 RAs combined was analyzed post hoc.
Results: There were 696,089 new users with 456,612 person-years of exposure to GLP-1 RAs. Baseline demographics, comorbidities, and use of other prescription medications in the 6 months before the index date were similar across medication cohorts. IRs (95% CIs) per 10,000 person-years were 1.0 (0.0-5.6) for lixisenatide, 6.0 (3.6-9.4) for exenatide, 5.1 (3.7-7.0) for liraglutide, 3.9 (3.1-4.8) for dulaglutide, and 3.6 (2.6-4.9) for semaglutide. The IRR (95% CI) for the anaphylaxis rate for the lixisenatide cohort compared with the pooled other GLP-1 RA cohort was 0.24 (0.01-1.35).
Conclusions: Anaphylaxis is rare with GLP-1 RAs. Lixisenatide is unlikely to confer higher risk of anaphylaxis than other GLP-1 RAs.
© 2024 by the American Diabetes Association.
Tags
Analytic: incidence | descriptive
Data Source: claims | multi-database
Research Focus: safety | diabetes
Study Design: observational | cohort study
Funding Transparency
This work was possible through:
- No Public Funding Sought or Awarded
Conflict of interest statement at time of publication:
M.S.A., C.B.J., K.J.R., C.W.S., and B.C. are employees of RTI Health Solutions. C.L.C., D.C.B., L.E.P., and S.L. are employees of Elevance Health. S.B., L.D., J.J., and C.P. are employees of Sanofi. V.R.A. has served as a consultant for Applied Therapeutics, Fractyl, Novo Nordisk, Pfizer, and Sanofi, conducting this work as a paid consultant to Sanofi, and is publishing in that capacity. V.R.A. has received research contracts (payable to institution) from Applied Therapeutics, Eli Lilly, Fractyl, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Entry last updated (DMY): 15-01-2025.