Provider Specialization in Inflammatory Bowel Diseases: Quality of Care and Outcomes
Published in Clinical Gastroenterology & Hepatology, December 1, 2024 | Online publication on June 4, 2024
Authors:
James D Lewis, Colleen M Brensinger, Lauren E Parlett![]() , Andres Hurtado-Lorenzo
, Andres Hurtado-Lorenzo![]() , Michael D Kappelman
, Michael D Kappelman![]()
DOI: 10.1016/j.cgh.2024.05.024 | Pubmed ID: 38844254
Abstract
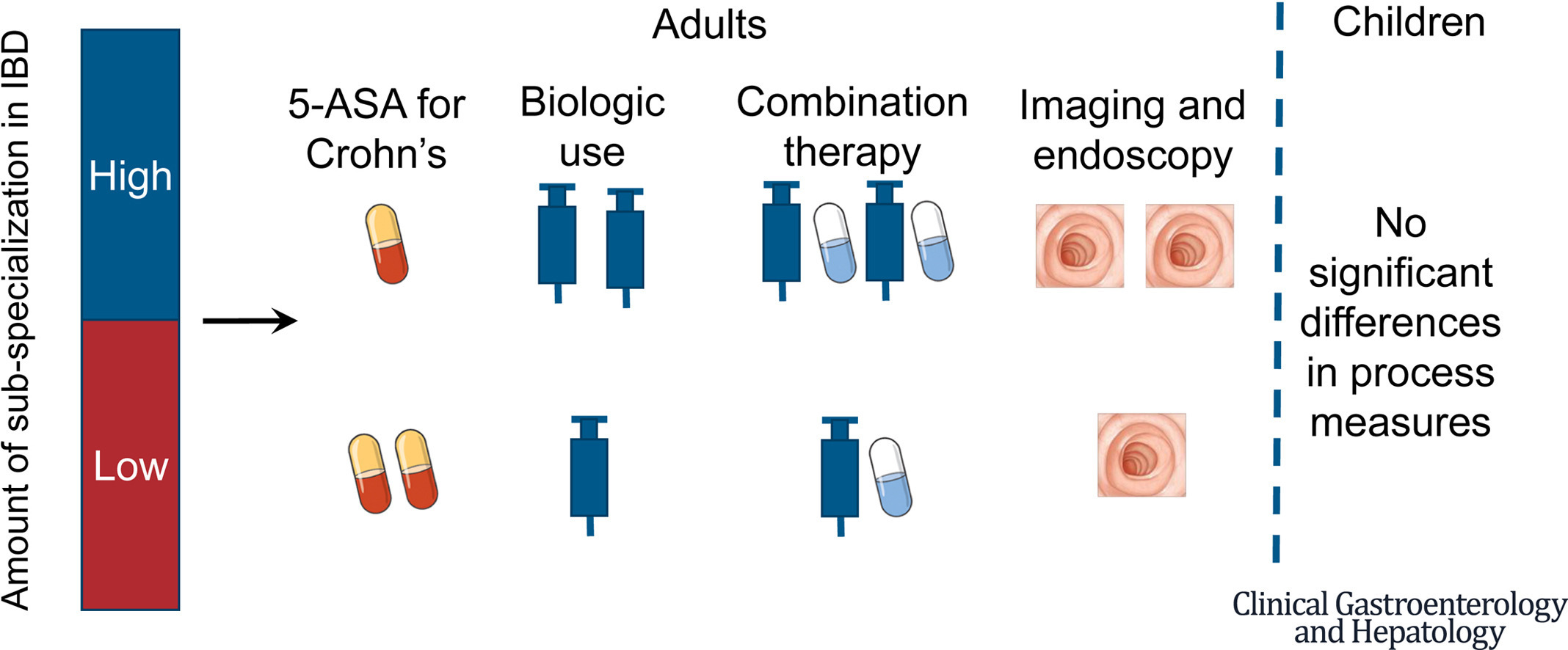
Background & aims: Management of inflammatory bowel diseases (IBD) is complex and variation in care has been well-documented. However, the drivers of practice variation remain unexplored. We examined variation based on the treating gastroenterologist's IBD focus (proportion of outpatient visits for IBD).
Methods: We conducted a retrospective cohort of newly diagnosed patients with IBD using data from Optum's deidentified Clinformatics Data Mart Database (2000-2020). The exposure variable was whether the treating gastroenterologist had an IBD focus (>90th percentile of IBD visits/total outpatient visits). We used adjusted regression models to evaluate associations between provider IBD focus and process measures (use of mesalamine, corticosteroid, biologic, and narcotic medications and endoscopic or radiographic imaging) and clinical outcomes (time to IBD-related hospitalization and bowel resection surgery). We tested for change in treatment patterns over time by including an interaction term for study era (2004-2012 vs 2013-2020).
Results: The study included 772 children treated by 493 providers and 2864 adults treated by 2076 providers. In children, none of the associations between provider focus and process or outcome measures were significant. In adults, care from an IBD-focused provider was associated with more use of biologics, combination therapy, and imaging and endoscopy, and less mesalamine use for Crohn's disease (P < .05 for all comparisons) but not with other process measures. Biologics were prescribed more frequently and narcotics less frequently during the later era (P < .05 for both). Hospitalization and surgery rates were not associated with IBD focus or era.
Conclusions: IBD care for adults varies by provider specialization. Given the evolving complexity, novel methods may be needed to standardize care.
Keywords: Biologic Therapy; Crohn’s Disease; Narcotics; Practice Volume; Steroids; Ulcerative Colitis.
Copyright © 2024 AGA Institute. Published by Elsevier Inc. All rights reserved.
Tags
Analytic: No tags set.
Data Source: claims
Research Focus: inflammatory bowel disease | provider specialty | quality of care
Study Design: cohort study
Funding Transparency
This work was possible through:
- Grant/Award
Additional details:
- Buse - NIH - UL1TR002489 : North Carolina Translational and Clinical Science Institute (NC TraCS)
- Hurtado-Lorenzo - CDC - 1U01DP006369 : Diversity within the Incidence, Prevalence, Treatment and Outcomes of Patients with IBD
- Wu - NIH - P30-DK050306 : Center for Molecular Studies in Digestive and Liver Diseases
Conflict of interest statement at time of publication:
These authors disclose the following: James D. Lewis consulted or served on an advisory board for Eli Lilly and Company, Samsung Bioepis, UCB, Bristol-Myers Squibb, Nestle Health Science, Merck, Celgene, Janssen Pharmaceuticals, Bridge Biotherapeutics, Entasis Therapeutics, AbbVie, Pfizer, Gilead, Galapagos, Sanofi, Arena Pharmaceuticals, Protagonist Therapeutics, Amgen, and Scipher Medicine; has had research funding from Nestle Health Science, Takeda, Janssen Pharmaceuticals, and AbbVie; has had educational grants from Takeda and Janssen; has performed legal work on behalf of generic manufacturers of ranitidine, including L. Perrigo Company, Glenmark Pharmaceuticals Inc, Amneal Pharmaceuticals LLC, Aurobindo Pharma USA, Inc, Dr. Reddy’s Laboratories, Inc, Novitium Pharma, Ranbaxy Inc, Sun Pharmaceutical Industries, Inc, Strides Pharma, Inc, and Wockhardt USA LLC; and owns stock in Dark Canyon Labs. Lauren E. Parlett has conducted studies on behalf of Sanofi and Pfizer, but they are not related to research presented in this manuscript. Michael D. Kappelman has consulted for AbbVie, Pfizer, Takeda, and Lilly; is a shareholder in Johnson & Johnson; and has received research support from Janssen and AbbVie. The remaining authors disclose no conflicts.
Entry last updated (DMY): 21-12-2024.