High-Risk Medications in Persons Living With Dementia: A Randomized Clinical Trial
Published in JAMA Internal Medicine, December 1, 2024 | Online publication on October 21, 2024
Authors:
Sonal Singh![]() , Xiaojuan Li, Noelle M Cocoros
, Xiaojuan Li, Noelle M Cocoros![]() , Mary T Antonelli
, Mary T Antonelli![]() , Ramya Avula, Sybil L Crawford
, Ramya Avula, Sybil L Crawford![]() , Inna Dashevsky, Hassan Fouayzi
, Inna Dashevsky, Hassan Fouayzi![]() , Thomas Harkins, Kathleen M Mazor, Ashley I Michnick
, Thomas Harkins, Kathleen M Mazor, Ashley I Michnick![]() , Lauren E Parlett
, Lauren E Parlett![]() , Mark Paullin, Richard Platt, Paula A Rochon, Cassandra Saphirak
, Mark Paullin, Richard Platt, Paula A Rochon, Cassandra Saphirak![]() , Mia Si, Yunping Zhou, Jerry Gurwitz
, Mia Si, Yunping Zhou, Jerry Gurwitz
DOI: 10.1001/jamainternmed.2024.5632 | Pubmed ID: 39432286
Abstract
Importance: Individuals with Alzheimer disease (AD) and Alzheimer disease-related dementias (ADRD) may be at increased risk for adverse outcomes relating to inappropriate prescribing of certain high-risk medications, including antipsychotics, sedative-hypnotics, and strong anticholinergic agents.
Objective: To evaluate the effect of a patient/caregiver and prescriber-mailed educational intervention on potentially inappropriate prescribing to patients with AD or ADRD.
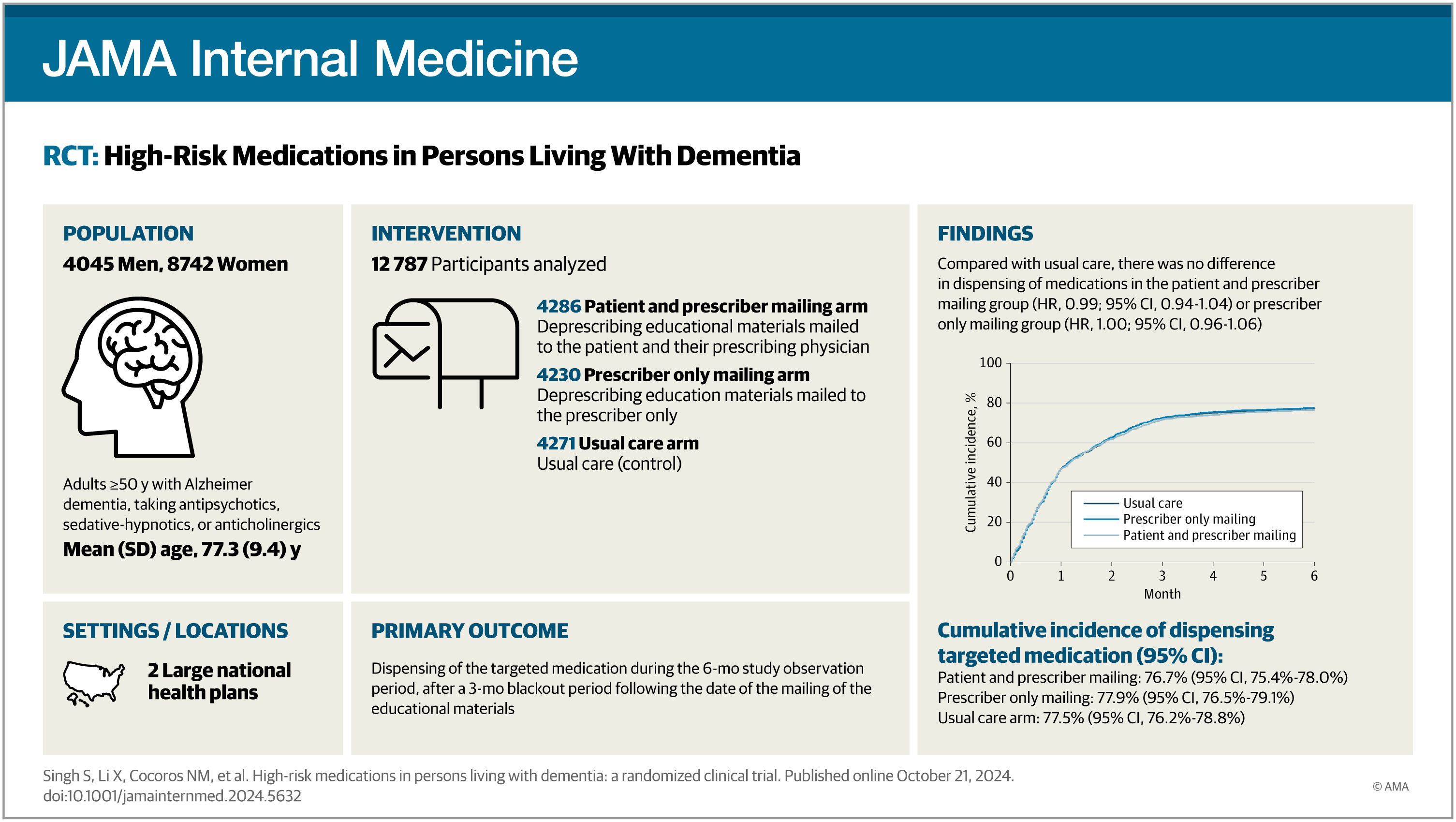
Design, setting, and participants: This prospective, open-label, pragmatic randomized clinical trial, embedded in 2 large national health plans, was conducted from April 2022 to June 2023. The trial included patients with AD or ADRD and use of any of 3 drug classes targeted for deprescribing (antipsychotics, sedative-hypnotics, or strong anticholinergics).
Interventions: Patients were randomized to 1 of 3 arms: (1) a mailing of educational materials specific to the medication targeted for deprescribing to both the patient and their prescribing clinician; (2) a mailing to the prescribing clinician only; or (3) a usual care arm.
Main outcomes and measures: Analysis was performed using a modified intention-to-treat approach. The primary study outcome was the dispensing of the medication targeted for deprescribing during a 6-month study observation period. Secondary outcomes included changes in medication-specific mean daily dose and health service utilization.
Results: Among 12 787 patients included in the modified intention-to-treat analysis, 8742 (68.4%) were female, and the mean (SD) age was 77.3 (9.4) years. The cumulative incidence of being dispensed a medication targeted for deprescribing was 76.7% (95% CI, 75.4-78.0) in the patient and prescriber mailing group, 77.9% (95% CI, 76.5-79.1) in the prescriber mailing only group, and 77.5% (95% CI, 76.2-78.8) in the usual care group. Hazard ratios were 0.99 (95% CI, 0.94-1.04) for the patient and prescriber group and 1.00 (95% CI, 0.96-1.06) for the prescriber only group compared with the usual care group. There were no differences between the groups for secondary outcomes.
Conclusions and relevance: These findings suggest medication-specific educational mailings targeting patients with AD or ADRD and their clinicians are not effective in reducing the use of high-risk medications.
Trial registration: ClinicalTrials.gov Identifier: NCT05147428
Tags
Analytic: survival analysis
Data Source: claims | intervention | sentinel common data model
Research Focus: deprescribing
Study Design: pragmatic clinical trial
Funding Transparency
This work was possible through:
- Grant/Award
Additional details:
- Gurwitz - NIH - 5R33AG069794 : Developing a PRogram to Educate and Sensitize Caregivers to Reduce the Inappropriate Prescription Burden in Elderly with Alzheimer's Disease Study (D-PRESCRIBE-AD)
- Gurwitz - NIH - R61AG069794 : Developing A Program To Educate And Sensitize Caregivers To Reduce The Inappropriate Prescription Burden In Elderly With Alzheimer's Disease Study (D-PRESCRIBE-AD)
Entry last updated (DMY): 21-12-2024.